12. Equilibriums with Complex Ions - Stability Constants
In complex ions, a central metal cation is covalently bound (coordinated) to a number of ligands (either neutral molecules or negative ions). Examples are Ag(NH3)2+, CdCl42-, Cu(NH3)42+, Zn(OH)42-, Fe(CN)63-, ...
The number of ligands that is directly attached to the metal ion is called the coordination number.
According to lewis-acid-base theory, the complex ion is formed by a combination reaction between the cation that acts as an electron pair acceptor and the ligands that act as electron pair donors. In aqueous solutions the metal ions can be considered as complex ions in which the metal ion is attached to a number of water molecules that act as ligands, e.g. Ni(H2O)62+(aq). This complex ion is also hydrated. Because the exact number of water molecules that is bound is usually unknown, these complex ions are denoted as Mn+(aq).
When ligands are added to the solution that form stronger covalent bonds with the cation than water, the water molecules can be displaced:
Cu(H2O)42+(aq) + 4 NH3(aq)  Cu(NH3)42+(aq) + 4 H2O(vl)
Cu(NH3)42+(aq) + 4 H2O(vl)
This reaction can be simplified to:
Cu2+(aq) + 4 NH3(aq)  Cu(NH3)42+(aq)
Cu(NH3)42+(aq)
The formation of a complex ion occurs in several subsequent steps.
The equilibrium constants for the formation of metal complexes from their constituent metal ions and ligands are called stability constants.
When we consider the global equilibrium
Ag+(aq) + 2 NH3(aq)  Ag(NH3)+(aq)
Ag(NH3)+(aq)
then the global equilibrium condition is given by:
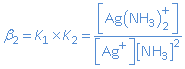
with b2 the global stability constant.
Summary