13. Influence of Complex Formation on the Solubility
Following Le Chatelierís principle, the solubility of a slightly soluble salt will increase when a ligand is added to the solution that preferentially forms a complex ion with the cation.
Example
AgCl is a slightly soluble salt. In the presence of NH3, the Ag+-ion can form the complex ion Ag(NH3)2+. The following equilibriums need to be considered:
When NH3 is added, reaction (b) will occur and [Ag+] will decrease. As a consequence equilibrium (a) will shift to the right: AgCl(s) dissolves.
For the global reaction
AgCl(s) + 2 NH3(aq)  Ag(NH3)2+(aq) + Cl-
Ag(NH3)2+(aq) + Cl-
we then have:
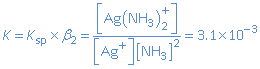

Summary