11. Influence of pH on Solubility
When the slightly soluble salt contains an anion that hydrolyses in water, the solubility of the salt will depend on the pH of the solution. These anions are the conjugate base of a weak acid, examples are CO32–, S2–, PO43–, F– , ...
Example
Solubility of metal sulfides as a function of the pH of the solution. In analytical chemistry, this pH dependence of the solubility of metal sulfides (in general denoted as MS(s)) is used for the separation of metal ions from a mixture. The following equilibriums must be considered:
A combination of the expressions (2) and (3) gives, after rearrangement:
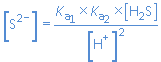
and the solubility S of the metal sulfide MS is given by:
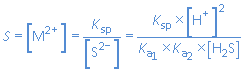
For H2S, the maximum solubility in water equals 0.1 mol/L . When an aqueous solution of a metal ion is saturated with H2S, then [H2S] = 0.1 mol/L and constant. The solubility of MS is determined by the value of its Ksp and the solution pH. For separation of metal ions in a mixture, the pH of the solution must be adjusted so that the sulfide of one metal precipitates completely while the sulfides of the other metal ions remain dissolved.

Summary