7. Calculating Solubility Product from Solubility Data
When the solubility S of a slightly soluble salt is experimentally determined, it is possible to calculate the value of Ksp. The solubility equals the amount of salt (in g or in mole) dissolved in 1 L of a saturated solution at the specified temperature.
Let us consider the salt MpLq(s) with a solubility of S mol/L:
MpLq(s)  p Mm+(aq) + q Ln-(aq)
p Mm+(aq) + q Ln-(aq)
In the saturated solution of MpLq:
[Mm+] = p x S and [Ln-] = q x S.
So:
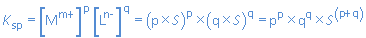

Summary