8. Calculating Solubility from Solubility Product
For any ionic compound with general formula MpLq , solubility product Ksp and solubility S (mol/L) we have:
MpLq(s)  p Mm+(aq) + q Ln-(aq)
p Mm+(aq) + q Ln-(aq)
For every MpLq-particle going into solution, p Mm+-ions and q Ln--ions are formed.
So:
[Mm+] = p.S and [Ln-] = q.S
When we combine this with the expression for Ksp:
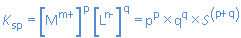
we find:
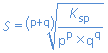

Summary