AgCl(s)  Ag+(aq) + Cl-(aq) Ag+(aq) + Cl-(aq)
with
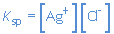
the equilibrium constant.
Ksp is called the solubility product.
The expression for the solubility product of any salt MpLq(s) can be derived by considering the equilibrium constant for its dissolution in water:
MpLq(s)  p Mm+(aq) + q Ln-(aq) p Mm+(aq) + q Ln-(aq)
and
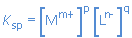
|