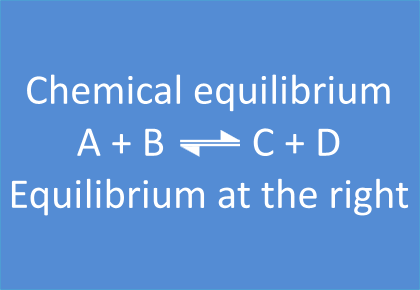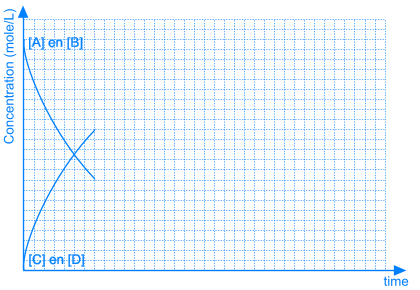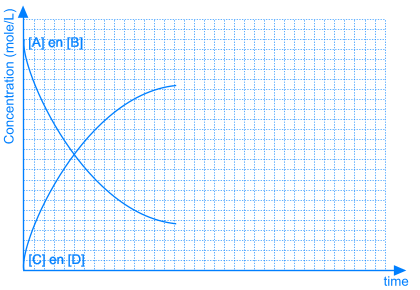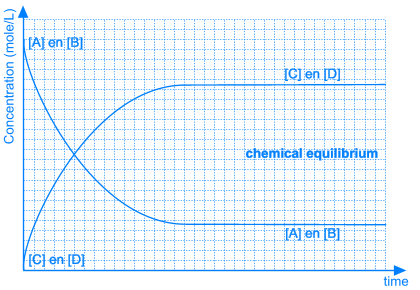
3. Position of the Equilibrium
In some equilibrium reactions the equilibrium state is reached when only a small fraction of the reactants has been converted into products. In that case we say that the equilibrium position lies to the left side. A reaction that does not occur at all, can be considered as a chemical equilibrium reaction where the equilibrium position lies completely to the left side.
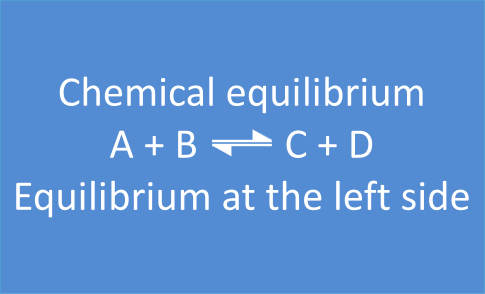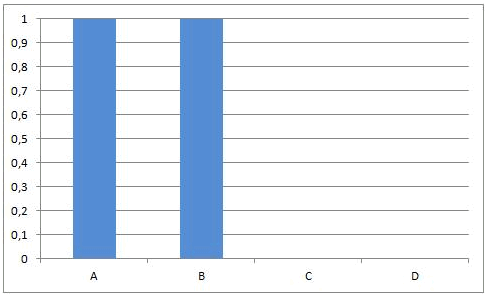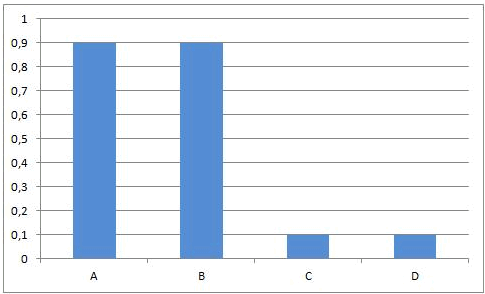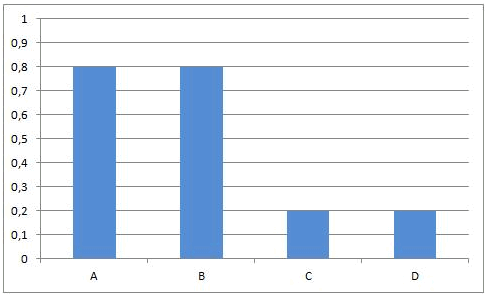
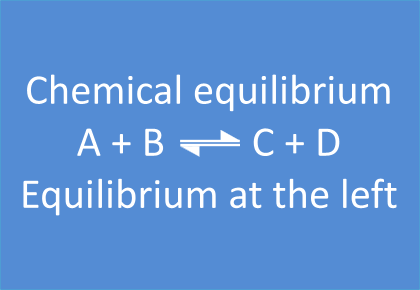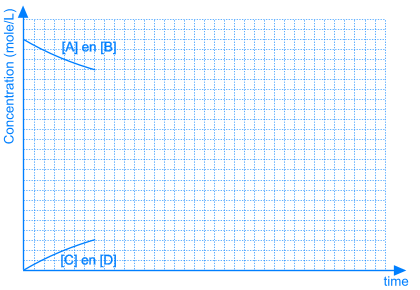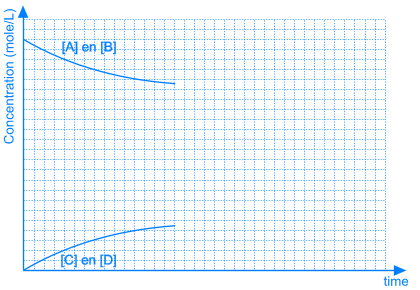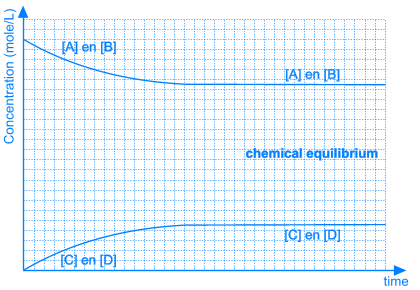
In other equilibrium reactions the equilibrium state is reached when nearly all the reactants have been converted into products. In that case we say that the equilibrium position lies at the right side. A reaction that goes to the completion, can be considered as a chemical equilibrium reaction where the equilibrium position lies completely at the right side.
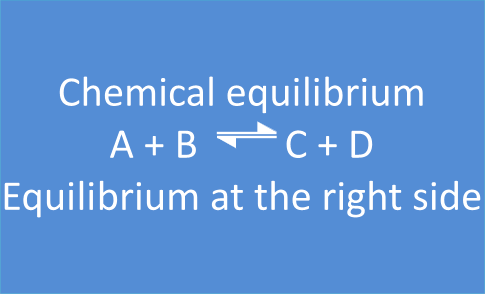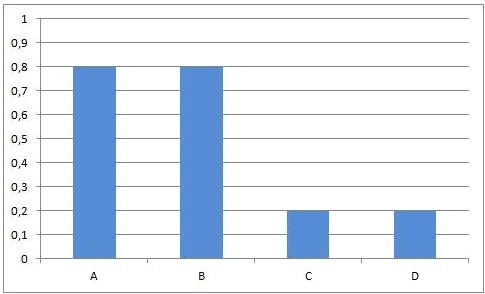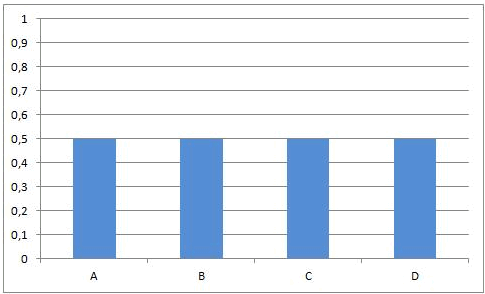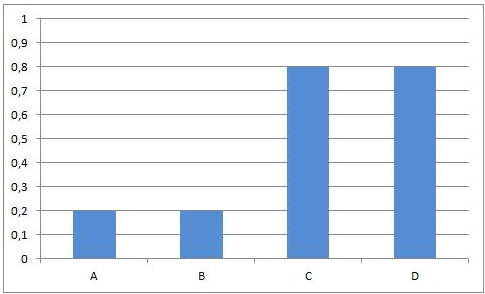
Summary
Position of the Equilibrium State |
Can be completely to the left, completely to the right, or on any position in between |
Is different for every equilibrium reaction |