2. Chemical Equilibrium
A lot of reactions seem to reach a standstill even before all the reactants are consumed.
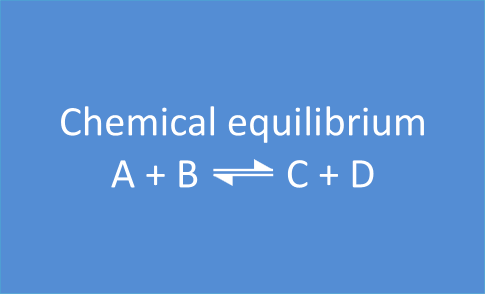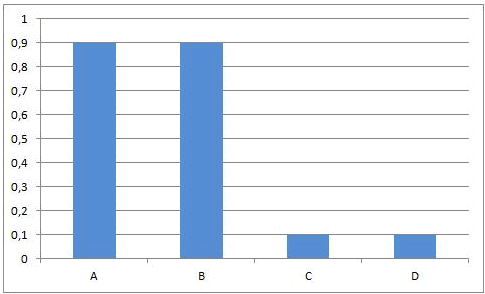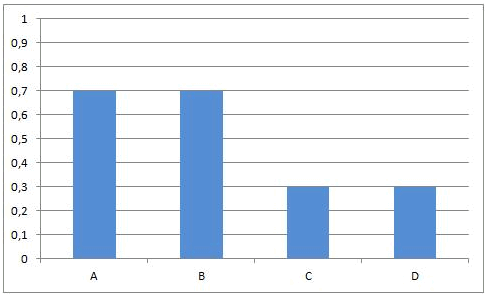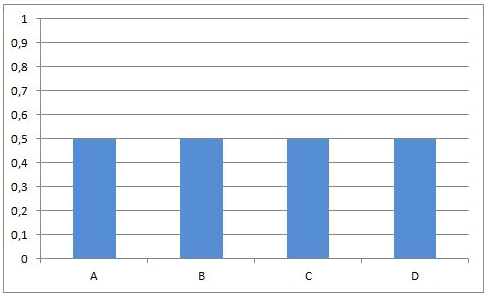
Moreover, mixing C and D (in stead of A and B) will lead to the same situation.
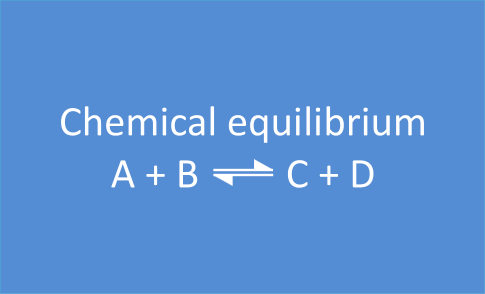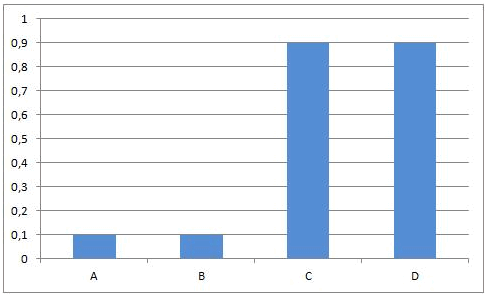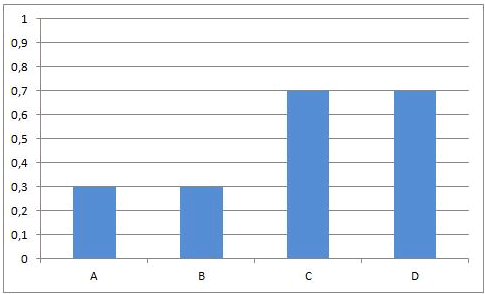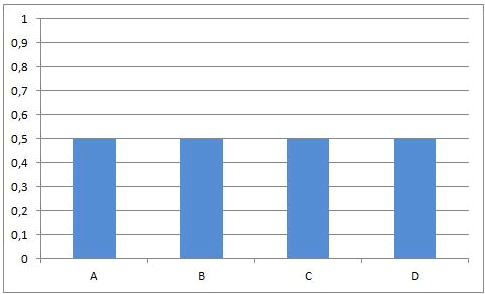
Those reactions are called equilibrium reactions. They are presented on the following way, with a bidirectional arrow:
A + B  C + D
C + D
The reaction can proceed in both direction:
1 |
forward reaction |
A + B  C + D C + D |
2 |
reverse reaction |
C + D  A + B A + B |
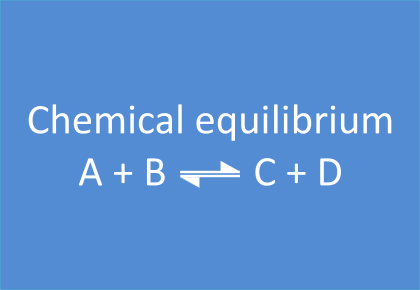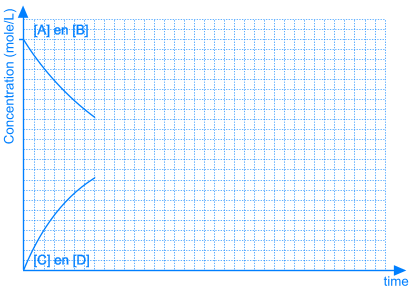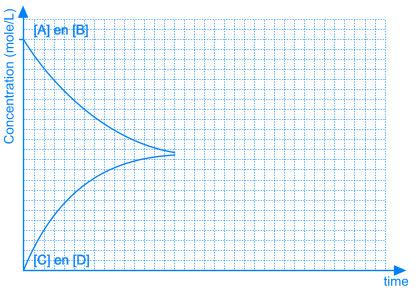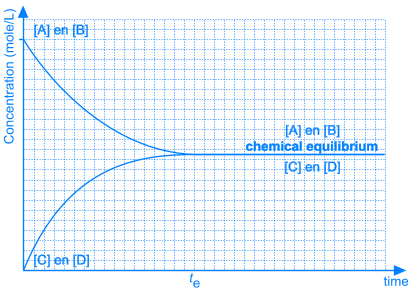
At the beginning of this reaction, when mixing A and B, the concentrations of A and B are maximal. The rate of the forward reaction at that time also is maximal (reaction rate is directly proportional to concentration(s) of reactants). Since there is no C and D formed at that time, the rate of the reverse reaction is equal to zero.
As A and B react, the concentrations of those components are decreasing and the same happens to the rate of the forward reaction. At the same time, more and more C and D is being formed, so the concentrations of C and D are increasing and the same happens to the rate of the reverse reaction.
Necessarily, this will lead to a situation were the rates of both the forward and reverse reactions will have the same value. From that moment on, the concentrations of A, B, C en D will stay constant: chemical equilibrium.
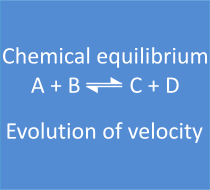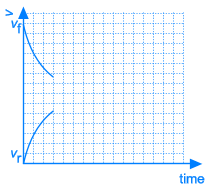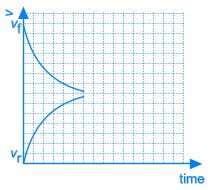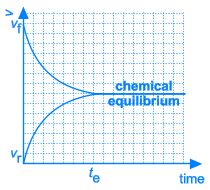
Summary
Equilibrium Reactions |
Seem to reach a standstill even before all the reactants are consumed |
The reverse reaction leads to the same situation |