A chemical reaction proceeds to completion, until one of the reactants (or all the reactants) is (are) consumed.
The reaction
A + B  C + D
C + D
will stop when all A and/or B is converted into C and D.
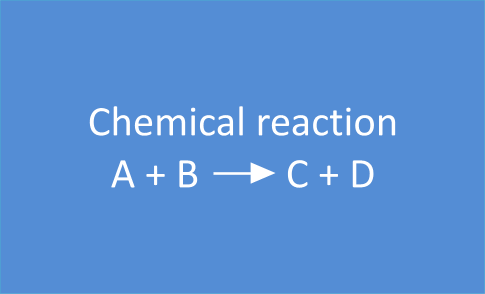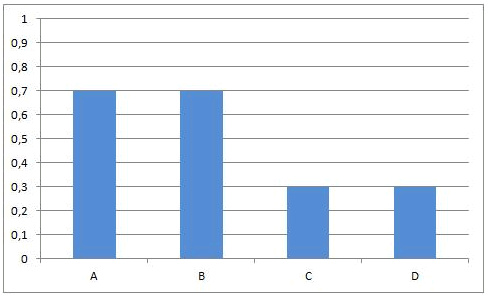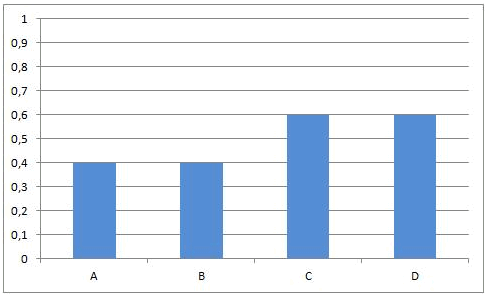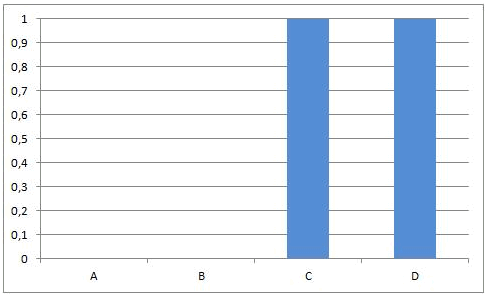
Reactions of this kind are called completed reactions.
When C and D are put together, there will be no reaction.
Some reacties can be extremely fast, even explosive.
Nitrogentrijodide decomposes into dinitrogen and dijodine:
2 NI3(s)  N2(g) + 3 I2(s)
N2(g) + 3 I2(s)
The reaction, initiated by a simple touch, occurs explosively.
Summary
Completed Reactions |
Proceed until one or all reactants is/are consumed |
Reverse reaction mostly impossible |