7. pH of a Solution of a Strong Base
When a strong base like NaOH is dissolved in water, nearly all NaOH-units will accept a proton:
NaOH(aq) + H2O(l)  Na+.H2O(aq) + OH-(aq)
Na+.H2O(aq) + OH-(aq)
So [OH-] will be equal to the original concentration of the base NaOH:
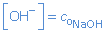
For instance: 0.1 mol/L NaOH(aq)  [OH-] = 0.1 mol/L
[OH-] = 0.1 mol/L  pOH = -log 0.1 = 1
pOH = -log 0.1 = 1  pH = 13.
pH = 13.
In this case the amount of hydroxide ions contributed by water is negligible compared with the 0.1 mol/L from the dissociation of NaOH. In pure water this amount is only 10-7 mol/L. Due to the presence of an abundance of hydroxide ions delivered by the strong base NaOH, the water equilibrium will shift completely to the left.
Summary
pH of a solution of a strong base B in water
|
[OH-] = coB
Calculate pOH
Calculate pH
|
