6. pH of a Solution of a Strong Acid
When a strong acid like HCl is dissolved in water, nearly all HCl-molecules will donate a proton:
HCl(aq) + H2O(l)  H3O+(aq) + Cl-(aq)
H3O+(aq) + Cl-(aq)
or
HCl(aq)  H+(aq) + Cl-(aq)
H+(aq) + Cl-(aq)
So [H3O+] or [H+] will be equal to the original concentration of the acid HCl:
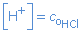
For instance: 0.1 mol/L HCl(aq)  [H+] = 0.1 mol/L
[H+] = 0.1 mol/L  pH = -log 0.1 = 1.
pH = -log 0.1 = 1.
pH + pOH = 14  pOH = 13.
pOH = 13.
In this case the amount of hydrogen ions contributed by water is negligible compared with the 0.1 mol/L from the ionization of HCl. In pure water this amount is only 10-7 mol/L. Due to the presence of an abundance of hydrogen ions delivered by the strong acid HCl, the water equilibrium will shift completely to the left.
Summary
|
pH of a solution of a strong acid HA in water
|
[H+] = coHA
Calculate pH
|
