5. Water: Acid or Base?
When an acid is dissolved in water, the following protolysis reaction will occur:
HA(aq) + H2O(l)  H3O+(aq) + A-(aq)
H3O+(aq) + A-(aq)
In this reaction water behaves like a base: it accepts the hydrogen ions donated by the acid HA.
When a base is dissolved in water, the following protolysis reaction will occur:
A-(aq) + H2O(l)  HA(aq) + OH-(aq)
HA(aq) + OH-(aq)
In this reaction water behaves like an acid: it donates the hydrogen ions accepted by the base A-.
So water can react as an acid (donate protons) and as a base (accept protons). That is why we call it an ampholyte.
The autoionization reaction of water:
H2O(l) + H2O(l)  H3O+(aq) + OH-(aq)
H3O+(aq) + OH-(aq)
can be seen as the protolysis reaction of the acid H2O, as well as the protolysis reaction of the base H2O.
Hence, the equilibrium constant of this reaction can be seen as the acid dissociation constant of water as well as the base dissociation constant of water:
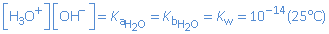
These equilibrium constants are very small. So the equilibrium state is positioned completely at the left. Only theoretically water is an acid/base, in practice, we consider water as a neutral particle.
Summary
Water is an Ampholyte
H2O(l) + H2O(l)  H3O+(aq) + OH-(aq) H3O+(aq) + OH-(aq) |
Water is a very weak acid
Ka = 10-14 |
Water is a very weak base
Kb = 10-14 |