7. Concentrations and Partial Pressures
For chemical equilibria in (aqueous) solutions we write concentrations (mol/L) in the expression of the equilibrium constant, called Kc.
A(aq) + B(aq)  C(aq) C(aq) |
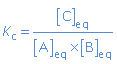 |
For chemical equilibria in the gaseous phase we can write concentrations (mol/L - Kc) or partial pressures (bar - Kp) in the expression of the equilibrium constant.
Relationship between Kc and Kp
a A(g) + b B(g)  c C(g) + d D(g) c C(g) + d D(g) |
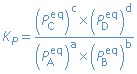 |
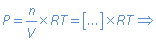
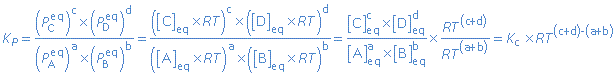
Activities
The concentrations and partial pressures in the equilibrium constant are in fact activities. The results of this assignment are:
The equilibrium constant is dimensionless.
In chemical equilibria in solutions, the concentration of the solvent (even if it is involved in the reaction) does not occur in the expression for the equilibrium constant, because it's activity is constant.
HOAc(aq) + H2O(l)  H3O+(aq) + OAc-(aq) H3O+(aq) + OAc-(aq) |
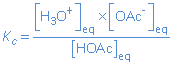 |
In heterogenous chemical equilibria in solutions, the concentrations of the undissolved components do not occur in the expression for the equilibrium constant, because their activities are constant.
AgCl(s)  Ag+(aq) + Cl-(aq) Ag+(aq) + Cl-(aq) |
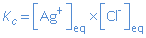 |
In heterogenous chemical equilibria in the gaseous phase, the concentrations/partial pressures of the non-gaseous components do not occur in the expression for the equilibrium constant, because their activities are constant.