5. Equilibrium Constant K
The progress of a chemical reaction can be expressed by the reaction quotient Q.
a A + b B  c C + d D
c C + d D
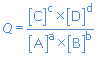
A equilibrium reaction proceeds until Q reaches a certain value (typical for that reaction). That value is called the equilibrium constant K of the reaction.
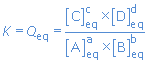
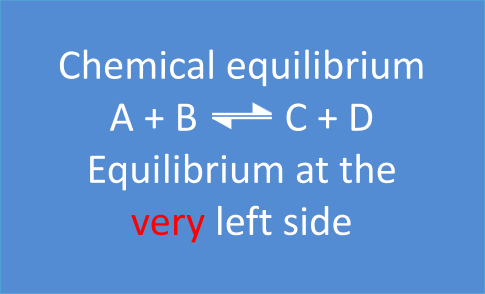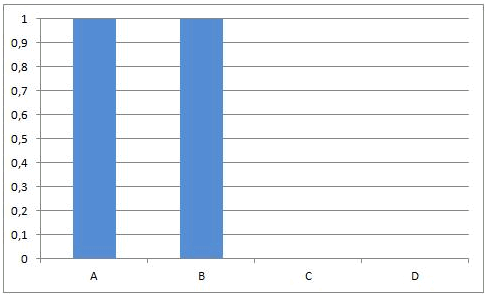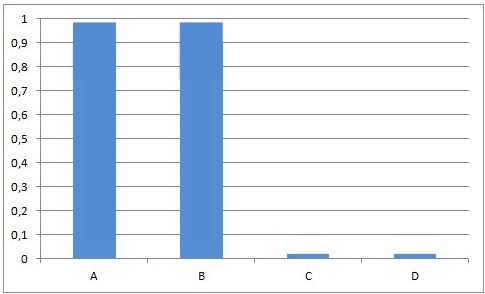
If the equilibrium constant K of an equilibrium reaction is small, the equilibrium state is positioned at the left. In this equilibrium state there are still lots of reactants A and B present and there are only small amounts of C and D. When K is very small (near to zero) it seems as if the reaction does not occur (concentrations of A and B allmost equal to the initial concentrations, concentrations of C and D allmost equal to zero).
If the equilibrium constant K of an equilibrium reaction is large, the equilibrium state is positioned at the right. In this equilibrium state there are only small amounts of reactants A and B present and there are large amounts of C and D. When K is very large (near to infinity) it seems as if the reaction proceeds to completion (concentrations of A and B allmost zero, concentrations of C and D allmost equal to maximal values).

Summary
K |
Examples |
Position of equilibrium state |
very small |
K < 10-30 |
completely at the left, reaction does not occur |
small |
10-30 < K < 10-3 |
at the left |
average |
10-3 < K < 103 |
in the middle, real chemical equilibrium |
large |
103 < K < 1030 |
at the right |
very large |
K > 1030 |
completely at the right, reaction proceeds to completion |