
Because [H+] and [OH-] are mostly very small, Sørensen suggested to represent the acidity of a solution in a more convenient way by using the pH-scale.
pH = - log [H+] and pOH = - log [OH-]
[H+] |
pH |
[OH-] |
pOH |
10-7 |
7 |
10-7 |
7 |
10-5 |
5 |
10-9 |
9 |
10-9 |
9 |
10-5 |
5 |
A similar scale is used voor equilibrium constants:
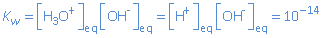
pKw = - log Kw= - log 10-14 = 14
Note that pH decreases as [H+] increases and vice versa!
Solution |
pH |
pOH |
neutral |
7 |
7 |
acidic |
< 7 |
> 7 |
basic |
> 7 |
< 7 |
Also note that, in any aqueous solution
pH + pOH = 14